Calcium Carbonate S Calcium Oxide S Carbon Dioxide G
100g of CaCO344g of CO2. CaCO3s CaOs CO2g H 178 kJ How many grams of CaCO3s would be made to react if 630 kJ of energy were provided.
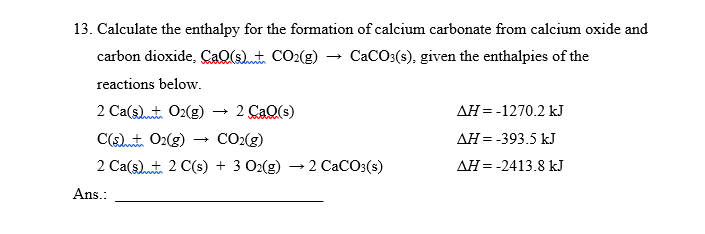
Solved 13 Calculate The Enthalpy For The Formation Of Chegg Com
Decomposing calcium carbonate yields calcium oxide and carbon dioxide.

. Now you know that your initial sample contained 20 impurities. H 2 O l Carbon dioxide diffuses into the. Limewater reacts with carbon dioxide gas to form solid calcium carbonate according to the following equation.
CaCO 3 s CaOs CO 2 g Calcium oxide also known as quicklime is a key ingredient in. The present calcium carbonate will react as shown in the equation below. 5 In general nitric oxide synthase NOS activity is regulated by a variety of mechanisms including cytoskeletal dynamics 6 and protein-protein.
Added hydrochloric acid calcium chloride carbon dioxide and waterFAQwhen calcium carbonate added hydrochloric acid calcium chloride carbon dioxide and wateradminSend emailDecember 21 2021 minutes read You are watching when calcium carbonate added. Chemistry questions and answers. When calcium carbonate is heated it breaks down to calcium oxide and carbon dioxide.
If we measure the amount of carbon dioxide generated by stoichiometry we can determine the amount of calcium carbonate. If the reaction produced 02960 moles of carbon dioxide then it must have consumed 02960 moles of calcium carbonate. When calcium carbonate is heated it breaks down into calcium oxide and carbon dioxide.
02960moles CaCO3 1001 g 1 mole CaCO3 2963 g. CaCO 3s 2HCl aq CaCl 2s CO 2g H 2 O l 6 As the reaction proceeds the carbon dioxide leaves the reaction mixture. G of CO2440100 44g of CO2.
CaCO₃ s CaO s CO₂ g If you heat 25 g CaCo₃ approximately what volume of carbon dioxide will you make. Calcium carbonate CaCO3 or CCaO3 CID 10112 - structure chemical names physical and chemical properties classification patents literature biological. Use calcium carbonates molar mass to find how many grams of calcium carbonate would contain this many moles.
The following thermochemical equation is for the reaction of calcium carbonates to form calcium oxide and carbon dioxideg. CaCO3 s CaO s CO2 g If you heat 25 g CaCO3 approximately what volume of carbon dioxide will you make. CaOH 2aq CO 2g CaCO 3s a What was the source of carbon dioxide gas in step 7.
Calcium carbonate calcium oxide carbon dioxide. Assume the conditions of temperature and pressure are such that 1 mole of any gas will occupy 224 L. Calcium carbonate decomposes when heated to give calcium oxide and carbon dioxide.
CaCO 3 s CaOs CO 2 g It is not realistically possible to measure the enthalpy change of this reaction directly but using the fact that both calcium carbonate and calcium oxide react directly with hydrochloric acid a Hess law cycle can be constructed to give the enthalpy change of. Has proposed using alkaline earth metal oxide or ZnO to promote ZrO2 retention in the SiO2. Calcium carbonate scalcium oxide s carbon dioxide g liters carbon dioxide How many grams of phosp horus P are needed to completely consume 699 L of chlorine gas according to the following reaction at 25 C and 1 phosphorus P4 s chlorine g --phosphorus trichloride 1 grams phosphorus P Show Hint How.
Calculate the molar mass of CO2. Assume the conditions of temperature and pressure are such that 1 mole of any gas will occupy 224 L.

Question Video Identifying The Chemical Equation With State Symbols That Corresponds To A Chemical Statement Nagwa

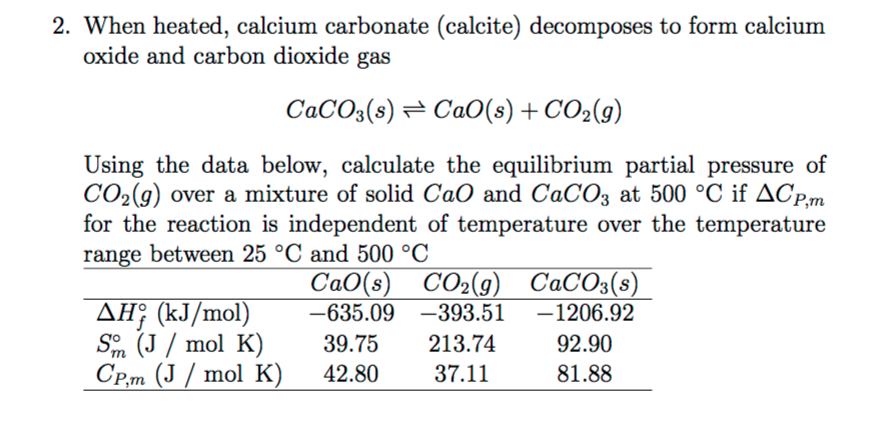
Comments
Post a Comment